
Materials:Mixtures, Solutions & Colloids Explained
Mixtures
A physical combination of two or more substances.
- Constituents retain their chemical properties
- Can be separated by physical methods
Types of Mixtures
- Solid + Liquid mixture
- Example: sugar in water, salt in oil
- Liquid + Liquid mixture
- Immiscible (do not mix): water + oil
- Miscible (mix uniformly): water + alcohol
- Solid + Solid mixture
- Example: clay and sand, alloys
- Solid + Gas mixture
- Example: smoke, dust, foam mattress
- Liquid + Gas mixture
- Example: fog, mist, clouds
- Gas + Gas mixture
- Example: air
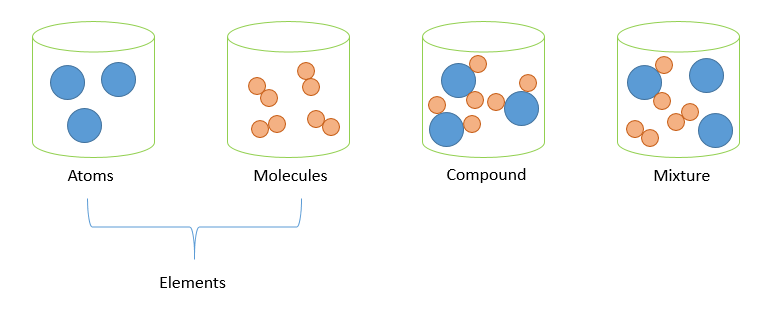
Classes of Mixtures
- Homogeneous mixture → uniform composition (e.g., salt + water, alcohol + water)
- Heterogeneous mixture → non-uniform composition (e.g., sand + water, oil + water)
Differences Between Homogeneous and Heterogeneous Mixtures
|
Homogeneous |
Heterogeneous |
|
Uniform mixture |
Non-uniform mixture |
|
Constituents cannot be seen |
Constituents visible |
|
Solute dissolves completely |
Solute does not dissolve completely |
|
One phase |
Two or more phases |
|
Stable over time |
Constituents separate when undisturbed |
Solute
A substance that dissolves in a solvent.
- Soluble: sugar, salt, ethanol
- Insoluble: sand, chalk, oil
Factors Affecting Dissolution
- Smaller particle size → faster
- Stirring → speeds mixing
- Heating → increases rate
Solvent
The substance in which solutes dissolve.
- Usually present in larger quantity
- Water is called the universal solvent
Examples
|
Solvent |
Solute |
|
Water |
Sugar, salt, milk powder |
|
Turpentine |
Oil paint |
|
Petrol |
Grease, fat, wax |
|
Kerosene |
Coal tar |
|
Alcohol |
Chlorophyll, dye |
|
Soap |
Fat |
|
Benzene |
Rubber |
Differences Between Solvent and Solute
|
Solvent |
Solute |
|
Dissolves the solute |
Dissolves in solvent |
|
Larger quantity |
Smaller quantity |
|
Usually liquid or gas |
Solid, liquid, or gas |
|
Lower boiling point |
Higher boiling point |
Solution
A homogeneous mixture of two or more substances.
- Solute dissolves completely
- Constituents do not separate
Examples:
- Salt + water
- Sugar + water
- Vinegar + water
- Alcohol + water
Types of Solutions
- Dilute solution → less solute
- Concentrated solution → more solute but can still dissolve more
- Saturated solution → maximum solute dissolved
Suspension
A heterogeneous mixture where solute does not dissolve in the solvent.
- Particles visible to the naked eye
- Particles settle when undisturbed
Examples:
- Gari + water
- Muddy water
- Oil + water
Differences Between Solution and Suspension
|
Solution |
Suspension |
|
Homogeneous |
Heterogeneous |
|
Particles not visible |
Particles visible |
|
One phase |
Two or more phases |
|
Solute dissolves |
Solute does not dissolve |
|
Cannot be filtered |
Can be filtered |
Colloid
A mixture in which one substance is permanently dispersed in another medium.
- Stable, particles do not dissolve
- Too small to see with naked eye
- Appears homogeneous but is heterogeneous
Examples:
- Milk
- Starch in water
- Blood
- Egg
- Toothpaste
Differences Between Colloid and Suspension
|
Colloid |
Suspension |
|
Homogeneous in appearance |
Heterogeneous |
|
Particles do not settle |
Particles settle |

